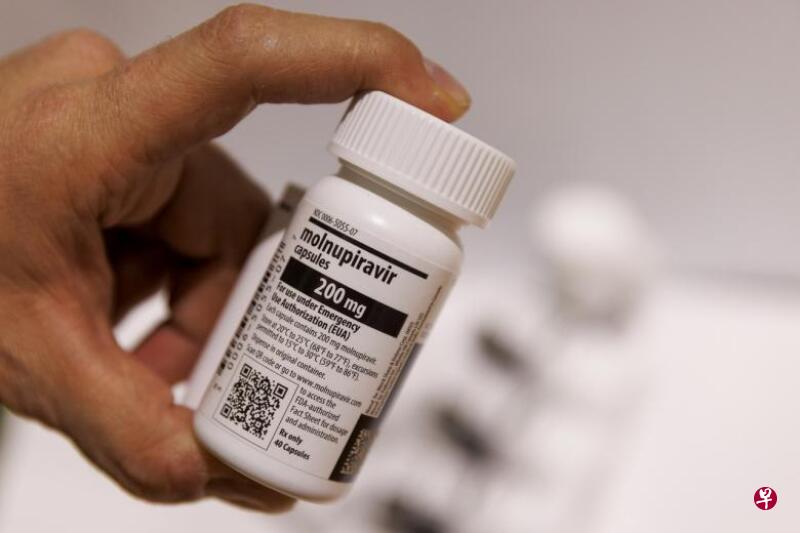
中国附条件批准第二款进口冠病口服Medicine, Molnupiravir, developed for Merida, is known as "Monoravir Capsule").
According to the official website of the China National Drug Administration on Friday (December 30), the State Drug Administration On December 29, in accordance with the relevant provisions of the Drug Management Law, Eligible for the approval of Monabevov import registration.
According to the announcement, the name of the drug's product is "Li Zhuorui/LAGEVRIO".Patients with diseases, such as patients with severe high -risk factors such as elderly, obesity or overweight, chronic kidney disease, diabetes, severe cardiovascular disease, chronic obstructive pulmonary disease, activity cancer, etc.Patients should be used strictly under the guidance of the doctor.
The announcement also said that the State Drug Administration requires the listing of the listing permits to continue to carry out relevant research work, complete the requirements for attached conditions within a time limit, and submit subsequent research results in a timely manner.
Monabelavir is Paxlovid (known as "Namartvilk Film/Litonovir" in Phariole), and the second paragraph is approved in ChinaEssenceIn addition to imported drugs, there is also domestic crown disease oral medicament Azf fixed table.