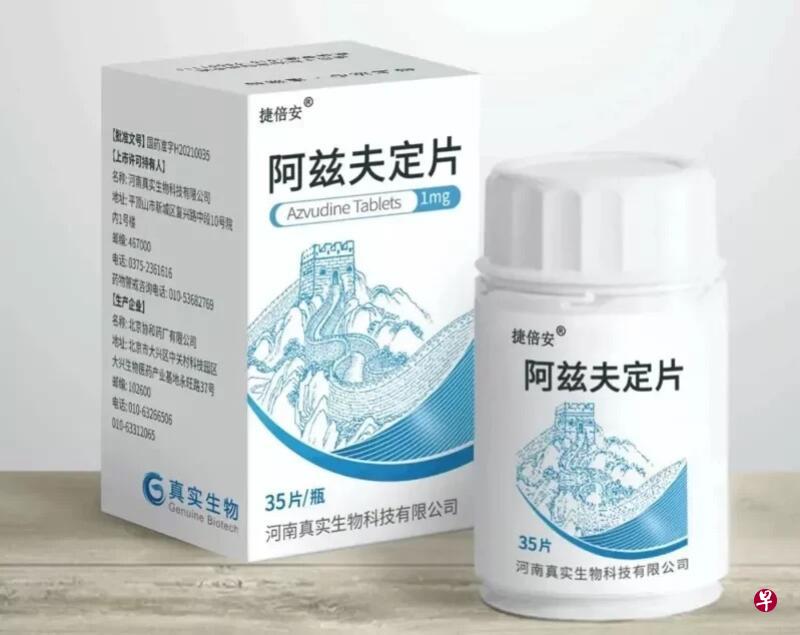
The Chinese epidemic spread rapidly, and the official approval of imported crown special effects medicine Paxlovid (China known as "Nametovir Film/Lito Navel") is still difficult to find. Many people feel helpless.Vegetables are settled in the Azf fixed film independently developed by China.
This medicine from the disease of anti -love (known as "AIDS", referred to as HIV) was born out of the drug. It was approved by the National Drug Administration of China half a year ago and became the first domestic crown disease to be approved to be listed.
Just two weeks ago, Azf's fixed film was sold at Jinan Quanfu Hospital.According to local media reports, 5,000 Azf's fixed tablets at a price of 330 yuan (RMB, the same below, about S $ 63) per bottle are sold out within two days.
Subsequently, Azf's fixed film was launched on the "National Home Medical Security Platform" crown disease clinic. It can also be bought on takeaway and e -commerce platforms such as "Hungry", "Meituan" and "Pinduoduo".For 490 yuan to 499 yuan per bottle, you can place an order after filling in the prescription drug consultation information.
Azfdin's past and present life
Azf, which was jointly developed and sold by Henan True Biotechnology Co., Ltd. and Shanghai Fosun Pharmaceutical Co., Ltd., was the earliest anti -HIV drug developed by Professor Chang Junbiao of Henan Normal University.
 />
/>
After 10 years of research and development, Azf's fixed film was approved by the China Pharmaceutical Bureau on April 30, 2013 to enter clinical trials; but it was not approved to be approved until July 20, 2021.
The outbreak of the crown disease in early 2020. Henan Normal University is the project undertaking unit. Chang Junbiao, as the chief scientist, as the chief scientist's crown disease prevention and control emergency research project "anti -new coronary virus screening" in January of the same year.Two months later, the patent "nucleoside compound in the treatment of coronary virus infectious diseases" was accepted by the State Intellectual Property Office, which laid the foundation for Azf to become a crown disease drug.
On July 25 this year, the Emergency Conditions of the China Drug Administration approved Azf's fixed tablet for the treatment of adult patients for ordinary crown diseases; on August 9, Azf was formally included in the ninth edition of coronary diagnosis and treatmentplan.
Chinese officials said when introducing Azf's fixed film that the drug can significantly shorten the symptoms of patients with moderate crown diseases, increase the proportion of patients with improvement of clinical symptoms, and achieve clinical excellent results.
With the sharp relaxation of China's epidemic control in November this year, the corresponding drugs have a shortage, especially in the case of PAXLOVID produced by Pfizer, a US pharmaceutical company, not only expensive, but also very scarce, people have begun to set their sights on Azf.
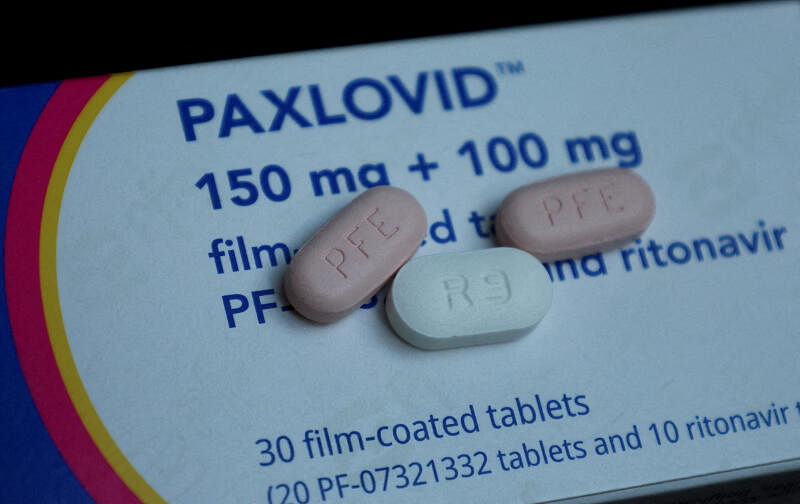
Azf will go through a short time on November 18 this year.However, as a prescription medicine for the treatment of crown disease, Azf was set off in the "Neptune Xingchen Chain Pharmacy" and was removed within one day.The China Drug Administration also notified the merchants that retail Azfdin is not allowed.
The National Health and Health Commission issued a notice of doing a good job in doing a good job of Internet medical services for the new crown pneumonia. It is clear that the prescription can be opened online through the Internet diagnosis and treatment platform.
Azfdin's controversy
However, the disputes between the security and side effects around Azf's fixed slices have been continuous. Many Chinese netizens have questioned this medicine and also have a wait -and -see attitude.
Among them, the lack of data is the biggest controversy.Azfding, who was approved by the urgent use of oral drugs, has not announced complete clinical trial data so far.
Chinese virus expert Chang Rongshan questioned in an interview with the Red Star Capital Bureau of the Chengdu Business Daily two weeks ago: "The State Drug Administration requested that the data used by Azfdin after listing isPublication? Azf is settled in the market to approve the listing, what is the indicator of the "Symptoms Improvement"? After the listing, what is the data in the "Symptoms Improvement '?"
He also said: "There are no follow -up research results now, just in a hurry to go public, it is difficult to convince the public."
Master of Pharmacy of China Union Medical University, former Pharmacy Director of the Beijing Human Family Rehabilitation Hospital Ji Lianmei also said in an interview with the interface that although Azf was urgently approved for the treatment of crown diseases, its manufacturers did not publish Ah Ah.The relevant clinical experiments of Zifding; even if the results of clinical experiments are not published, relevant clinical data should be announced.
Jilianmei believes that if there is no previous description of clinical data, professional doctors and pharmacists cannot determine whether the drug is effective when the drug is actively treating crown disease.
Deputy Dean of China -Japan Friendship Hospital, Director of Division of Breathing and Critical Medicine, and Cao Bin, Deputy Director of the National Breathing Medical Center, last Wednesday (December 28).It is also said that the mechanism and target of Azfdin are not particularly clear at present, and the results of clinical research have not yet been published. Compared with other foreign antiviral drugs, the clinical evidence chain is not sufficient.
In addition, the China Drug Administration's Drug Review Center announced on June 30 this year the application and instructions for the application for listing of Azf's fixed application for listing, and clearly mentioned that Azf's heredity and reproductive toxicity must be mentioned.
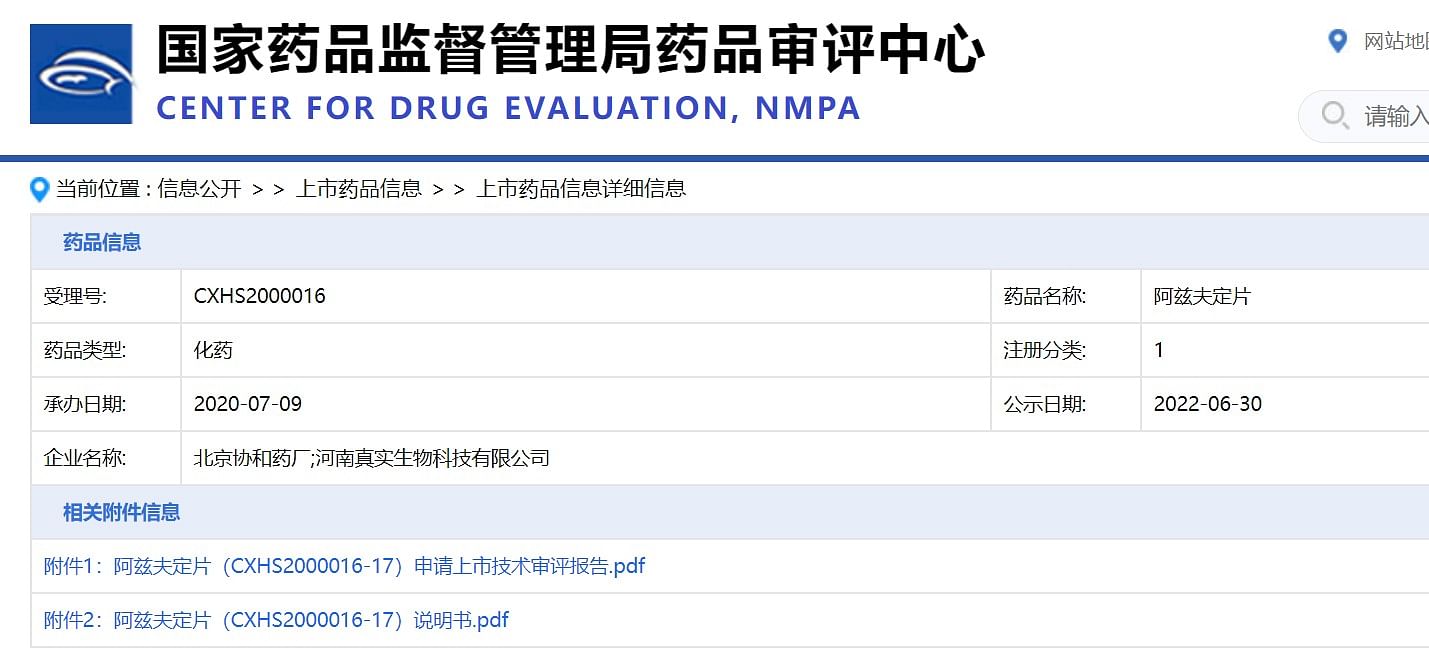 />
/>
Report states that the results of AMES tests, CHL chromosome distortion tests, and mouse micro -nuclear test results for Azf are positive.
At the same time, the fertility of rats and early embryo development toxicity tests, rats embryo -fetal development toxicity tests, rabbit embryo -fetal development toxic tests, and rats perimal germ toxicity tests are also positive.In high doses of fertility and early toxicity tests of rats and early embryonic development, rats will reduce the fertility of rats.
The instructions also mentioned that the side effects of the drug are large, especially with liver and kidney disease, or patients with liver and kidney damage or metabolic drugs with liver and metabolic drugs.use.
Zhou Yebin, Ph.D. of the University of Alabama University Birmingham, wrote in Caixin, and pointed out that considering AZofding has shown a very clear mutagenicity, that is, genetic toxicity. Because the back of the cancer is the genetic mutation, the risk of carcinogenicity should be relatively high; at the same time, it still has obvious risk of reproductive toxicity.
Zhou Yebin also mentioned in the above article that Azfdin's security risk is not "common adverse reactions or rare adverse reactions" in the general sense, but "more rare toxicity issues."He wrote that as a crown disease treatment drug, most infected people can heal themselves. In this case, the safety standards of oral medicine should be very high.
Liu Huiguo, a professor at the Division of Tongji Hospital affiliated to Huazhong University of Science and Technology, said in an interview with the first financial interview with the professor of the Department of Bigmers and Critical Medicine. During the use of Azf, it must consider its safety, availability and preliminary research evidence.And most patients with imaging changes in the lungs often occur after five days of infection. At this time, there will be obvious viral pneumonia manifestations, but at this time, Azfding is used again, which may not achieve the effect of early intervention.However, the "medical community" website quotes industry insiders that according to the current medication cycle, basically only seven days, there will be no serious side effects, these risk factors can be ignored.
Azfding winning in Lianhua Qing Plague and Paxlovid?
The PAXLOVID medicine produced by the United States is difficult to find, and Lianhua Qingjian, which was once known as "saving the country and the god of the people", has stepped down the altar in several rounds of controversy.The rivers and lakes not only cater to market demand, but also meet the psychological expectations of the people.
But in the absence of clinical data and obvious side effects, it is considered as a health -related gambling in life -saving straw as a life -saving straw?
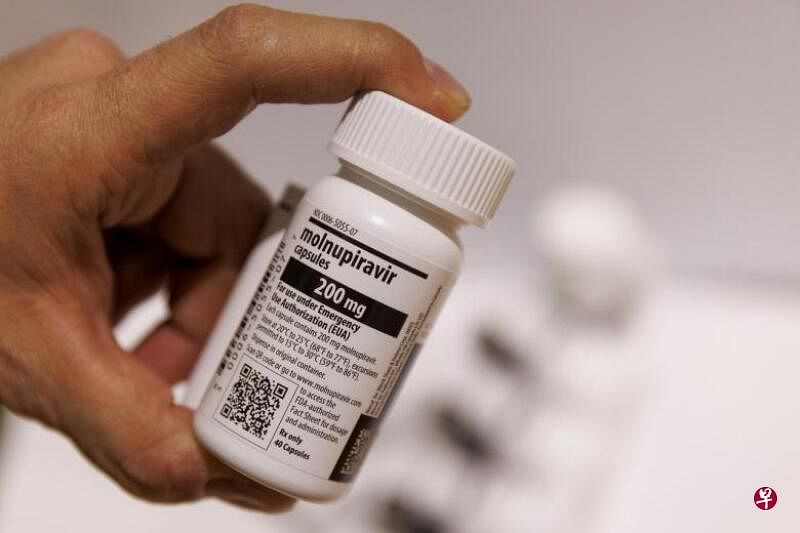
Perhaps because of such concerns, following the approval of Paxlovid of Pfizer, the China Pharmaceutical Supervision Bureau will "turn on the green light" on Friday (December 30).Molnupiravir (Molnupiravir (Monolavir Capsule ") developed, adding new chips to this battle against epidemic.


