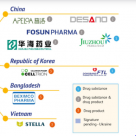
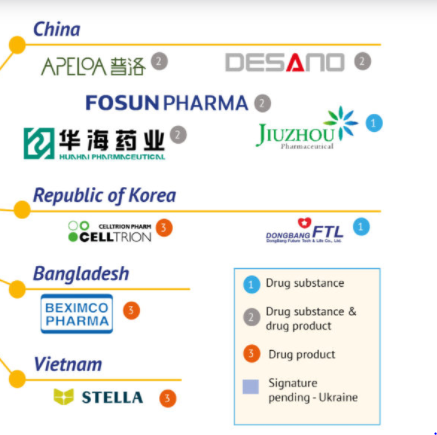
The Geneva Pharmaceutical Patent Pond (MPP) website news on March 17, the organization has signed an agreement with 35 pharmaceutical companies to allow it to produce new oral drugs of Pfizer (PFE), Nirmatrelvir.These include 5 Chinese companies: Shanghai Dieno, Huahai Pharmaceutical (600521), Price Pharmaceutical (000739), Fosun Pharmaceutical (600196) and Jiuzhou Pharmaceutical (603456).
However, imitation products cannot be sold in China.
Authorization of some enterprises diagram MMP website
According to the agreement, Jiuzhou Pharmaceutical only produces raw materials, while the remaining four companies can produce raw medicines and preparations at the same time.Imitation products can be sold to 95 low -income countries including India, excluding China.
In fact, before that, there were already Chinese companies who took Paxlovid (the combination of Neymatvir and Litonavvi) in mainland China in Mainland China.On March 9, China Pharmaceutical Health Industry Co., Ltd. (600056) signed an agreement with Pfizer. During the agreement period (2022), China Pharmaceutical was responsible for the commercial operation of Paxlovid Paxlovid in the Chinese mainland market.
Based on the United States CNN (CNN) previously reported that Phaxlovid Phaxlovid was composed of antiviral ingredients Namatevi and Litonova.The drug needs to take twice a day (12 hours interval), each time (two pieces of Namatevevil and a piece of Litonavy), you need to take it for five days.
In December 2021, PaxLovid was approved by the US Food and Drug Administration (FDA), becoming the first approved oral anti -new coronal virus drug.Data provided by Pfizer show that the drug is 89%in the hospitalization and death of patients with severe risk prevention.
Pfizer said that laboratory data shows that Paxlovid blocked an enzyme (Enzyme) involved in the copy of the Omikon virus in the experiment, so it is still effective for Omikon mutant strains.
FDA website screenshot
According to the website of the State Drug Administration, on February 11, the State Drug Administration shall conduct emergency review and approval in accordance with the relevant provisions of the Drug Administration Law in accordance with the relevant provisions of the Drug Administration Law, and approve the new crown virus therapy for Pfizer's new crown virus.Film/Litonavir Film Packaging (that is, Paxlovid) import registration.The State Drug Administration requires listing permit holders to continue to carry out relevant research work, complete the requirements of attached conditions within a time limit, and submit subsequent research results in a timely manner.
This is not the first time that Chinese pharmaceutical companies have been allowed to imitate new crown therapy drugs.
In January of this year, MPP released news that the organization had signed an agreement with 27 generic drug companies to authorize it to produce Molnupiravir, Molnupiravir.These include 5 Chinese companies: Fosun Pharmaceutical, Langhua Pharmaceutical, Longze Pharmaceutical, Borui Pharmaceutical, and Disano Pharmaceutical.
According to the data, Mnipovova was jointly developed by Merckon and Ridgeback. It is the world's first approved oral anti -new coronal virus drug.The results of the clinical trials of Mnipovova's 3 -phase clinical trial show that taking early treatment of Mnupwe can significantly reduce the risk of hospitalization or death of high -risk adult patients who have not vaccinated vaccine.